无论您是在概念,发展还是生产阶段,MW Life Sciences都专门从事帮助您将里程碑桥接到医疗设备市场。
Getting your product through the development and regulatory process can be quite a challenge. We can help. As a turnkey concept-to-supply partner, we provide a unique single source solution to medical device companies and entrepreneurs alike. Our innovative, high-value concept-to-supply services span design, development engineering, sourcing, assembly and packaging services.
我们的策略是提前参与并作为开发团队的延伸运营。我们通过从成立完成每个项目的初级工程,开发价值工程产品,通过向客户提供优质工程。我们拥有所有必要的资源,将我们的活动纳入客户的产品开雷竞技官网客服发和供应链流程。
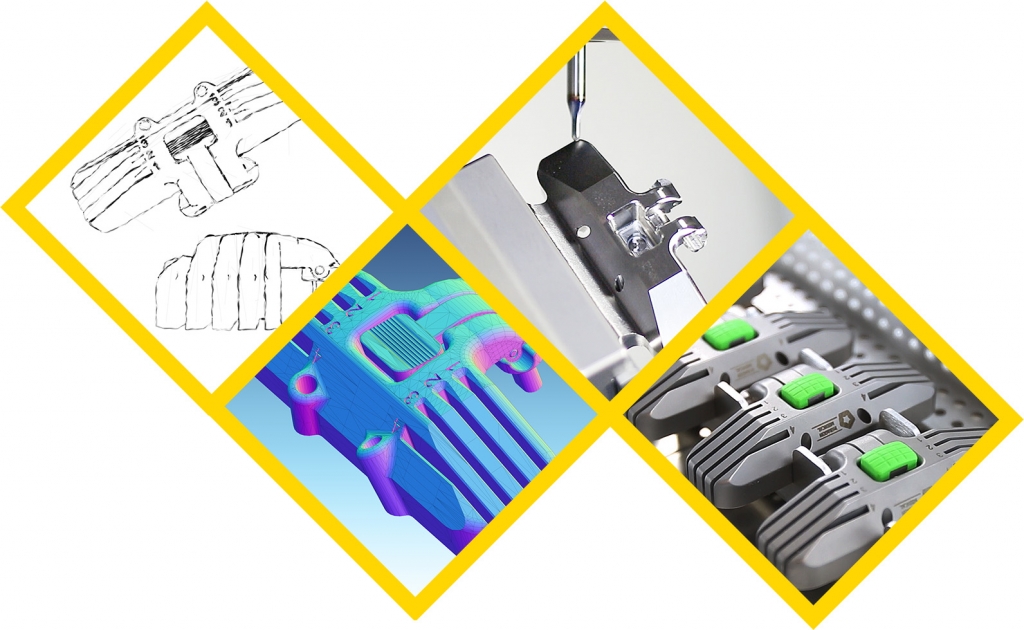
Working together, we establish common goals, and then achieve them. Our commitment to excellence is evident throughout the design, validation, and advanced manufacturing processes. We offer:
- Medical Device Product Development
- Contract Engineering for Medical Devices
- 医疗器械产品设计
- Contract Manufacturing for Medical Devices
我们总是记住你的终点线。我们对监管需求的理解以及整个商业化进程提供了客户,我们的产品将按时发布的信心。
您开发有多少产品?必须概念,原型录音和验证了多少乐器?让我们参与您的产品开发,我们可以帮助您在更短的时间内完成更多工作。
我们的团队通过整个商业化进程拥有广泛的项目管理经验。我们了解合规流程,包括监管和设计验证要求。此知识允许更无缝的设计转移以确保及时发布。
设计开发Service Benefits
降低制造成本
A key benefit is our new product introduction process at our global manufacturing sites. As part of that process, our Design for Manufacturability (DFM) capabilities can reduce complexity that results in the reduction of both cost and quality issues, while ensuring long-term manufacturability is evaluated early in the process.
我们作为业务研发部门的延伸,我们的可制造性服务设计有助于优化精密组件的制造。我们的开发团队在最新技术中良好,包括软件和设备,在制造先进,精密部件方面拥有重大经验。我们迅速发展了一丝细致的计划,最小化了步骤数量,但优化了物质产量。这包括用于公差和其他微小变化的建议,这提高了制造的缓和速度,同时确保了适当的功能。结果更快,更具成本效益的交付,而不会质量影响。
我们的能力涵盖广泛的医学device products and our team members have extensive experience in design and development for medical devices, giving us a first-hand understanding of the industry’s product development needs. We have worked with small to major OEMs on a variety of projects while maintaining strict confidentiality of every project.
加速市场时间并尽量减少风险
我们的交钥匙概念到供应商业模式加速了上市时间,并最大限度地减少客户的风险。随着ISO和QSR合规性,最先进的设施,竞争成本和既定的全球联络网络,在内的全球联系人,我们可以让您在那里。
In addition, we can assist in the prototyping stage of part development, helping you transition smoothly from raw concept to production run. We respond promptly to your needs and can adjust planning and execution rapidly to accommodate any changes that may arise.
加强知识产权并获得技术专长
我们知道我们不是唯一努力向前移动项目的资源。还有其他重要的功能可以在您的程序中产生差异。
我们为知识产权生成和景观提供技术评估和评估,竞争分析和服务。
We can help you strengthen your intellectual property portfolio or create a matrix of existing art to determine opportunities and points of weakness. Our industry experience ensures a thorough assessment and we can offer distribution of your company’s products across all medical device markets.
In addition, we provide design control, compliance related documentation tracking, regulatory filings and domestic and offshore sourcing of components and subassemblies.
Medical Device Design & Development Phases
我们的模块化服务提供了最大的灵活性。我们为客户提供完整的交钥匙方法或个人服务,以满足您的精确需求。通过一个概念来到我们,或在过程中的任何阶段输入。我们将获得您需要去的项目。
概念
This phase begins with a concept or problem. In Phase 1, we gain an understanding of the market requirements and design input. We brainstorm multiple concepts and then narrow them down. With access to rapid prototyping technology, we can put prototype devices into the hands of the end user for feedback on feasibility. We iterate working models and can support market research and focus groups as needed.
开发
开发了一个完整的项目计划,适用正式的设计控制。概念设计精制。我们继续设计迭代,建立详细的工程规范和公差。我们的工程师将启动对设计的危害分析,开始开发测试方法。在第2阶段结束时,已经选择了设计形式和测试的关键性能要求。
验证/验证
Test methods are finalized. Design verification testing ensures design performance meets design input objectives. Design validation testing is performed under defined operating conditions (either actual or simulated use) on initial production units or equivalents. Product packaging design is completed and biocompatibility testing performed.
Manufacturing scale-up
我们的扩展包括过程验证,过程能力和无菌验证。所有这些测试都是在完整的制造级别文档下构建的产品上进行的。供应商是合格的,生产设备安装和合格。在第4阶段完成之前满足保质期要求。建立最终文件,包括标签和使用说明。
全规模制造
所有监管批准都是担保和认真培训的工作人员实施您的产品。我们建立库存以支持产品发布,并及时对您的产品预测进行响应。
联系


